Aging is often perceived as a visible phenomenon: wrinkles, gray hair, and reduced physical endurance. However, the aging process begins at the cellular level long before these outward signs appear. Cellular aging refers to the gradual decline in the functionality of cells, which ultimately impacts tissue and organ performance. Unlike chronological age, which measures the number of years a person has lived, biological or cellular age reflects the true condition of cells and tissues.
Understanding cellular aging is critical because it directly influences longevity, disease susceptibility, and overall health. While chronological age is fixed, biological age can be influenced by lifestyle, diet, environment, and emerging therapeutic interventions [1]. By identifying the factors that accelerate or slow cellular aging, we can adopt strategies to maintain cellular health, enhance vitality, and potentially extend a healthy lifespan.
What Is Cellular Aging?
Cellular aging occurs when cells lose their ability to divide and function optimally due to accumulated damage over time. Normal cell aging is a part of life, but certain cells may enter a state called senescence, where they remain metabolically active but no longer divide. Senescent cells secrete inflammatory molecules that can damage neighboring cells, contributing to tissue decline.
Apoptosis, or programmed cell death, is another mechanism in cellular aging. It is a protective process that removes damaged or dysfunctional cells. However, excessive apoptosis can contribute to organ dysfunction, while insufficient apoptosis allows damaged cells to accumulate, increasing disease risk.
Biological Markers of Cellular Aging
Scientists use several markers to identify and measure cellular aging:
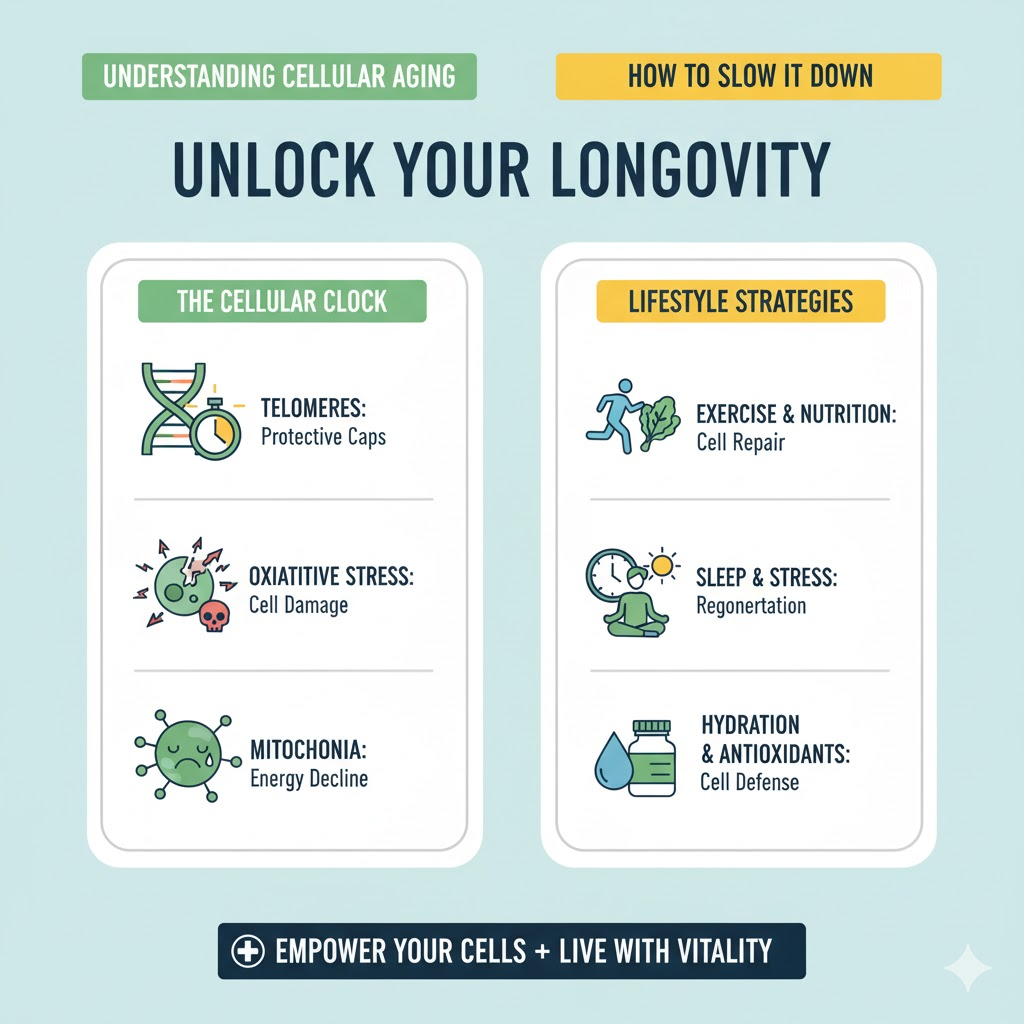
Telomere Shortening
Telomeres are protective caps at the ends of chromosomes. Each time a cell divides, telomeres shorten, eventually triggering senescence. Shorter telomeres are associated with aging-related diseases and reduced lifespan.
DNA Damage Accumulation
DNA molecules in cells are constantly subjected to damage from oxidative stress, radiation, and replication errors. Accumulated DNA damage impairs cell function and accelerates aging.
Mitochondrial Dysfunction
Mitochondria generate energy for cells, but with age, they become less efficient, leading to reduced energy production and increased reactive oxygen species (ROS).
Epigenetic Changes
Modifications to DNA or histones can alter gene expression without changing the genetic code. Epigenetic drift over time can disrupt normal cellular function.
Protein Degradation
Misfolded or damaged proteins accumulate in aged cells, impairing function and triggering inflammatory responses.
Causes of Cellular Aging
1. Oxidative Stress and Free Radicals
Reactive oxygen species (ROS) are highly reactive molecules produced during normal metabolism. In excess, ROS causes oxidative damage to DNA, proteins, and cell membranes, accelerating cellular aging [2]. Oxidative stress is implicated in numerous age-related diseases, including cardiovascular disease, neurodegeneration, and cancer. Antioxidants in food and the body neutralize ROS, but an imbalance leads to cellular damage.
2. Inflammation (Inflammaging)
Chronic low-grade inflammation, termed inflammaging, is a hallmark of aging. Senescent cells and oxidative stress promote the secretion of pro-inflammatory molecules, which impair tissue function and contribute to chronic diseases. Maintaining a healthy lifestyle and diet can reduce systemic inflammation and slow the aging process.
3. Lifestyle Factors
Lifestyle choices significantly influence the rate of cellular aging:
- Poor Diet: Excessive sugar, processed foods, and low nutrient intake accelerate oxidative stress and inflammation.
- Sedentary Behavior: Lack of exercise impairs mitochondrial function and reduces telomere length.
- Smoking and Alcohol: Both introduce toxins that increase oxidative stress and cellular damage.
- Stress: Chronic stress elevates cortisol levels, promoting inflammation and accelerating aging.
4. Genetic and Epigenetic Factors
While lifestyle matters, genes play a role in determining how fast cells age. Genetic variations can influence susceptibility to diseases, mitochondrial efficiency, and telomere length [3]. Epigenetic modifications, influenced by environment and behavior, further determine longevity and cellular resilience.
Effects of Cellular Aging on the Body
Cellular aging impacts every organ system and tissue in the body. As cells accumulate damage over time, their functionality declines, leading to noticeable physical, cognitive, and immune-related changes. Understanding these effects highlights why slowing cellular aging is crucial for long-term health.
1. Physical Health Implications
As cells age, their regenerative capacity diminishes. This affects the body in several ways:
- Muscle Mass Loss (Sarcopenia):
Aging muscle cells lose strength and volume, reducing overall mobility and increasing the risk of falls. Adults can lose up to 3–8% of muscle mass per decade after age 30 if no interventions are taken. Resistance training and protein-rich diets can mitigate this loss.
- Reduced Tissue Regeneration:
Wounds and injuries heal more slowly due to decreased cellular turnover and impaired stem cell function. This increases susceptibility to infections and prolonged recovery times.
- Organ Function Decline:
Organs like the heart, liver, and kidneys gradually lose efficiency. Reduced cardiac output, diminished liver detoxification, and lower kidney filtration capacity increase vulnerability to age-related diseases like heart disease, fatty liver, and chronic kidney disease.
2. Skin and Appearance
Cellular aging significantly impacts the skin, the body’s largest organ. Key changes include:
- Wrinkles and Fine Lines:
Collagen and elastin production declines with age, reducing skin elasticity. Combined with oxidative stress from environmental factors like UV radiation, wrinkles become more prominent.
- Loss of Skin Hydration:
Aging cells produce less hyaluronic acid, leading to dryness, thinning, and increased susceptibility to injury.
- Slower Healing:
Reduced cellular turnover delays wound closure, making skin more prone to infections and scarring.
- Pigmentation Changes:
Age spots and uneven pigmentation occur due to DNA damage in melanocytes, the cells responsible for skin color.
3. Cognitive Function
Neurons are highly sensitive to cellular damage because they rarely regenerate. Aging at the cellular level affects brain function in several ways:
- Memory Decline:
Oxidative stress and mitochondrial dysfunction impair neuronal communication, leading to forgetfulness and slower information processing [4].
- Neurodegenerative Risk:
Cellular aging increases susceptibility to diseases like Alzheimer’s, Parkinson’s, and other forms of dementia. Protein misfolding, accumulation of senescent glial cells, and chronic inflammation all contribute to these conditions.
- Cognitive Fatigue:
Reduced energy production in aging neurons results in mental fatigue, slower learning, and difficulty multitasking.
4. Immune System Decline
Aging cells compromise the body’s immune system, a phenomenon known as immunosenescence. Effects include:
- Reduced Infection-Fighting Ability:
Senescent immune cells fail to respond effectively to pathogens, increasing vulnerability to infections such as influenza, pneumonia, and COVID-19.
- Lower Vaccine Efficacy:
Older adults often produce weaker immune responses to vaccinations, making preventive measures less effective.
- Chronic Inflammation:
Senescent cells release inflammatory molecules, leading to low-grade chronic inflammation. This contributes to tissue damage, cardiovascular disease, and autoimmune conditions.
- Delayed Healing and Recovery:
Reduced immune cell function slows wound healing and prolongs recovery after illness or surgery.
Additional Systemic Impacts
Beyond these primary effects, cellular aging also influences:
- Metabolic Decline: Insulin sensitivity decreases, increasing the risk of type 2 diabetes and obesity.
- Cardiovascular Health: Oxidative stress and endothelial cell aging impair blood vessel function, raising blood pressure and increasing atherosclerosis risk.
- Hormonal Changes: Aging cells in endocrine glands reduce hormone production, affecting energy levels, bone health, and reproductive function.
How to Slow Cellular Aging
1. Diet and Nutrition
A nutrient-rich diet supports cellular repair and longevity:
- Antioxidant-Rich Foods: Berries, leafy greens, nuts, and seeds neutralize ROS.
- Omega-3 Fatty Acids: Found in fatty fish, they reduce inflammation and support brain and heart health.
- Polyphenols and Vitamins: Resveratrol, vitamin C, and E protect DNA and cellular membranes.
- Caloric Restriction & Intermittent Fasting: Shown to improve cellular repair, reduce oxidative stress, and extend lifespan in studies.
2. Physical Activity and Exercise
Exercise promotes mitochondrial health, telomere preservation, and cellular regeneration:
- Aerobic Exercise: Increases oxygen delivery, boosts mitochondrial function, and reduces oxidative stress.
- Resistance Training: Preserves muscle mass, stimulates protein synthesis, and maintains functional independence with age.
3. Stress Management
Chronic stress accelerates cellular aging. Practices that reduce stress include:
- Meditation and mindfulness.
- Yoga and deep-breathing exercises [5].
- Regular relaxation to reduce cortisol and systemic inflammation.
4. Sleep and Recovery
Deep sleep allows cellular repair and detoxification:
- Supports DNA repair and protein synthesis.
- Regulates circadian rhythms, enhancing hormonal balance and metabolic efficiency.
4. Supplements and Emerging Therapies
- Resveratrol, CoQ10, and NAD+ Boosters: Support mitochondrial function and DNA repair.
- Senolytics: Experimental therapies aimed at clearing senescent cells.
- Ongoing research explores drugs and nutraceuticals to slow or reverse cellular aging.
5. Avoiding Harmful Habits
- Stop smoking and limit alcohol.
- Minimize exposure to environmental toxins and pollutants.
- Avoid excessive sugar and processed foods that increase oxidative stress
Monitoring and Measuring Cellular Age
- Telomere Testing: Assesses the length of telomeres as a marker of cellular age.
- Biological Age Assessments: Combine multiple biomarkers to estimate cellular vs. chronological age.
- Wearables & Health Apps: Track heart rate variability, sleep quality, and activity levels, providing insights into cellular health and longevity metrics.
Conclusion
Cellular aging is the foundation of the aging process, affecting every tissue, organ, and function in the body. While we cannot stop time, science-backed strategies can slow cellular aging and improve longevity. A combination of nutrient-rich diets, regular physical activity, adequate sleep, stress management, and avoidance of harmful habits preserves cellular health. Emerging therapies like senolytics and supplements show promise for further enhancing longevity. By taking proactive measures, individuals can maintain vitality, prevent disease, and age healthily at the cellular level.
Looking for more health and wellness solutions ? Don’t miss these related guides:-
Frequently Asked Questions:
1. What is cellular aging, and how does it differ from normal aging?
Cellular aging refers to the decline in cell function over time, while normal aging includes visible signs and organ-level changes. Cellular aging drives the underlying biological decline.
2. What are the main causes of cellular aging?
Oxidative stress, chronic inflammation, poor lifestyle habits, and genetic/epigenetic factors are the primary causes of cellular aging.
3. How do telomeres influence the aging process?
Telomeres protect chromosomes; their shortening triggers cell senescence and contributes to aging and age-related diseases.
4. Can diet slow down cellular aging?
Yes. Antioxidant-rich foods, omega-3 fatty acids, polyphenols, and caloric moderation help reduce oxidative stress and support cellular repair.
5. What types of exercise are best for cellular health?
Aerobic exercise boosts mitochondrial function and oxygen delivery, while resistance training preserves muscle mass and stimulates protein synthesis.
References
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. https://www.cell.com/cell/fulltext/S0092-8674(13)00645-4
- Finkel, T., Holbrook, N. J. (2000). Oxidants, oxidative stress and the biology of aging. Nature, 408(6809), 239–247. https://www.nature.com/articles/35041687
- World Health Organization. (2023). Healthy aging and functional ability. https://www.who.int
- Harvard T.H. Chan School of Public Health. (2022). Antioxidants and aging. https://www.hsph.harvard.edu
- Campisi, J. (2013). Aging, cellular senescence, and cancer. Annual Review of Physiology, 75, 685–705. https://www.annualreviews.org/content/journals/10.1146/annurev-physiol-030212-183653